First principles calculations of the thermodynamic stability of Ba, Zr, and O vacancies in BaZrO3
Abstract
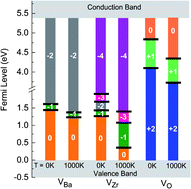
The temperature dependence of the stability of bulk BaZrO3 (BZO) and of the vacancies in this material are investigated by considering phonon contributions to the free energy. The stability diagram of BZO is determined for different chemical environments. With increasing temperature the stability region becomes smaller which is particularly caused by the strong temperature dependence of the chemical potential of gaseous oxygen. The free formation energy of Ba, Zr, and O vacancies in BZO is calculated for all possible charge states and for different atomic reservoirs. While the free formation energy of Zr vacancies is strongly influenced by temperature a weaker dependence is found for Ba and O vacancies. This also has an effect on the charge transition levels at different temperatures. The present results demonstrate that O poor reservoir conditions and a Fermi level close to the valence band maximum favour a high concentration of doubly positively charged O vacancies which is a prerequisite to get a large number of protonic defects and good proton conductivity. In such a chemical environment the number of Ba and Zr vacancies is low so that Ba and Zr deficiencies are not an important issue and BZO remains sufficiently stable.

 Please wait while we load your content...
Please wait while we load your content...