The selective hydrosilylation of norbornadiene-2,5 by monohydrosiloxanes†
Abstract
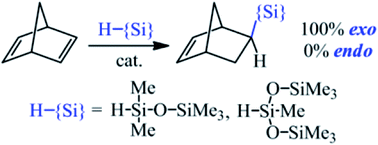
A simple one-step approach for the selective synthesis of exo-norbornenes with organosilicon substituents is suggested through the direct hydrosilylation of norbornadiene-2,5 with chlorine-free silanes. Using the example of norbornadiene-2,5 hydrosilylation with pentamethyldisiloxane and 1,1,1,3,5,5,5-heptamethyltrisiloxane, the possibility of obtaining exo-isomers of norbornenes with 100 exo-/endo-selectivity is shown. The investigation of Pt-, Rh-, and Pd-complexes in combination with various ligands as catalysts was performed. The hydrosilylation of norbornadiene-2,5 in the presence of Pt- or Rh-catalysts was not selective and led to a mixture consisting of three isomers (exo-/endo-norbornenes and substituted nortricyclane). In the case of the Pd-salt/ligand catalytic system, the formation of an endo-isomer was not observed at all and only two isomers were formed (exo-norbornene and nortricyclane). The selectivity of exo-norbornene/nortricyclane formation strongly depended on the nature of the ligand in the Pd-catalyst. The best selectivity was revealed when R-MOP was the ligand, while the highest catalytic activity was reached with a dioxalane-containing ligand.


 Please wait while we load your content...
Please wait while we load your content...