Coexistence of normal and inverse deuterium isotope effects in a phase-transition sequence of organic ferroelectrics†
Abstract
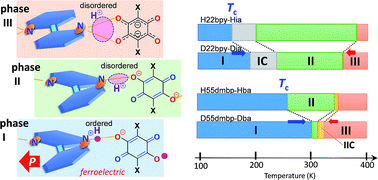
Supramolecular cocrystals of anilic acids with 2,2′-bipyridines exhibit successive phase transitions as well as unusual isotope effects. Ferroelectricity driven by a cooperative proton transfer along the supramolecular chains is accompanied by huge permittivity (a maximum of 13 000) at the Curie point, as well as a large spontaneous polarization (maximum 5 μC cm−2) and a low coercive field ranging from 0.5 to 10 kV cm−1. Deuterium substitutions over the hydrogen bonds smoothly raise the Curie point and simultaneously reduce other phase-transition temperatures by a few tens of degrees. The coexistence of opposite isotope effects reduces the temperature interval of the intermediate paraelectric phase from 84 to 10 K for the 5,5′-dimethyl-2,2′-bipyridinium bromanilate salt. The bipyridine molecules exhibit interplanar twisting, which represents the order parameter relevant to the high-temperature phase transitions. The normal and inverse temperature shifts are ascribed to the direct and indirect effects, respectively, of the lengthened hydrogen bonds, which adjusts the molecular conformation of the flexible bipyridine unit so as to minimally modify their adjacent intermolecular interactions.


 Please wait while we load your content...
Please wait while we load your content...