The versatile Co2+/Co3+ oxidation states in cobalt alumina spinel: how to design strong blue nanometric pigments for color electrophoretic display†
Abstract
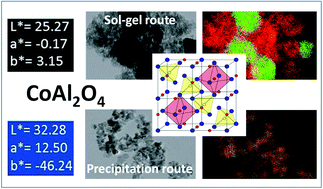
Blue cobalt inorganic pigments with spinel-type structure have been revisited in order to understand the origin of blackening at low temperatures and to design strong blue nanosized materials. Starting from a sol–gel process, the so-called Pechini route, the correlation between the structural features (inversion rate, Co over-stoichiometry, Co valence states) of the spinel network and its thermal history under air up to high temperatures (T = 1400 °C) allows concluding that the stabilization of CoIII in octahedral sites is at the origin of the blackening of the pigment annealed at low and medium temperatures. EELS coupled with TEM analyses (occurrence of multiple phases with various Al/Co atomic ratios) lead to us to conclude definitively about the variation of Co valence states. A top-down (mechanical grinding) and a bottom-up approach lead to the definition of a synthesis route (co-precipitation in basic medium followed by annealing at medium temperatures under Ar) allowing the design of strong blue pure nano-sized pigments to be incorporated in inks. Hybrid blue positively charged particles were mixed with white negatively charged particles to formulate dual-colour inks. A dual-colour display was filled with the as-prepared inks and tested under ±150 V.


 Please wait while we load your content...
Please wait while we load your content...