Discovery of novel 1,4-disubstituted 1,2,3-triazole phenylalanine derivatives as HIV-1 capsid inhibitors
Abstract
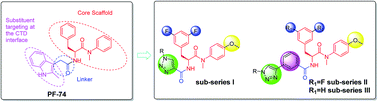
The HIV-1 capsid (CA) protein plays crucial roles in both early and late stages of the viral life cycle, which has intrigued researchers to target it to develop anti-HIV drugs. Accordingly, in this research, we report the design, synthesis and biological evaluation of a series of novel phenylalanine derivatives as HIV-1 CA protein inhibitors using the Cu(I)-catalyzed azide and alkyne 1,3-dipolar cycloaddition (CuAAC) reaction. Among this series of inhibitors, compound II-10c displayed a remarkable anti-HIV activity (EC50 = 2.13 μM, CC50 > 35.49 μM). Furthermore, surface plasmon resonance (SPR) binding assays showed that compounds II-10c and PF-74 (lead compound) have similar affinities to HIV-1 CA monomer. Further investigation showed that the weak permeability and water solubility of representative compounds were probably the important factors that restricted their cell-based activity. Preliminary structure–activity relationships (SARs) were inferred based on the activities of these compounds, and their known structure. The most promising new compound was studied with molecular dynamics simulation (MD) to determine the preferred interactions with the drug target. Finally, the activities of members of this series of inhibitors were deeply inspected to find the potential reasons for their anti-HIV-1 activity from various perspectives. This highlights the important factors required to design compounds with improved potency.
- This article is part of the themed collection: A Decade of Progress in Click Reactions Based on CuAAC


 Please wait while we load your content...
Please wait while we load your content...