ZnO decorated Sn3O4 nanosheet nano-heterostructure: a stable photocatalyst for water splitting and dye degradation under natural sunlight
Abstract
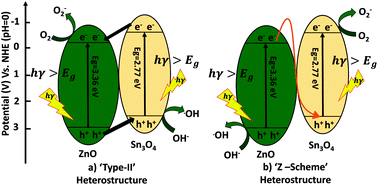
Herein, a facile hydrothermally-assisted sonochemical approach for the synthesis of a ZnO decorated Sn3O4 nano-heterostructure is reported. The phase purity of the nano-heterostructure was confirmed by X-ray diffraction and Raman spectroscopy. The morphological analysis demonstrated a nanosheet-like structure of Sn3O4 with a thickness of 20 nm, decorated with ZnO. The optical band gap was found to be 2.60 eV for the ZnO@Sn3O4 nano-heterostructure. Photoluminescence studies revealed the suppression of electron–hole recombination in the ZnO@Sn3O4 nano-heterostructure. The potential efficiency of ZnO@Sn3O4 was further evaluated towards photocatalytic hydrogen production via H2O splitting and degradation of methylene blue (MB) dye. Interestingly, it showed significantly superior photocatalytic activity compared to ZnO and Sn3O4. The complete degradation of MB dye solution was achieved within 40 min. The nano-heterostructure also exhibited enhanced photocatalytic activity towards hydrogen evolution (98.2 μmol h−1/0.1 g) via water splitting under natural sunlight. The superior photocatalytic activity of ZnO@Sn3O4 was attributed to vacancy defects created due to its nano-heterostructure.
- This article is part of the themed collection: Editors' Collection: Nanomaterials for the environment


 Please wait while we load your content...
Please wait while we load your content...