Sonochemistry-enabled uniform coupling of SnO2 nanocrystals with graphene sheets as anode materials for lithium-ion batteries†
Abstract
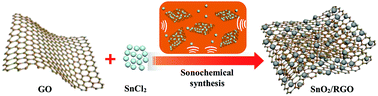
SnO2/graphene nanocomposite was successfully synthesized by a facile sonochemical method from SnCl2 and graphene oxide (GO) precursors. In the sonochemical process, the Sn2+ is firstly dispersed homogeneously on the GO surface, then in situ oxidized to SnO2 nanoparticles on both sides of the graphene nanosheets (RGO) obtained by the reduction of GO under continuous ultrasonication. Graphene not only provides a mechanical support to alleviate the volume changes of the SnO2 anode and prevent nanoparticle agglomeration, but also serves as a conductive network to facilitate charge transfer and Li+ diffusion. When used as a lithium ion battery (LIB) anode, the SnO2/graphene nanocomposite exhibits significantly improved specific capacity (1610 mA h g−1 at 100 mA g−1), good cycling stability (retaining 87% after 100 cycles), and competitive rate performance (273 mA h g−1 at 500 mA g−1) compared to those of bare SnO2. This sonochemical method can be also applied to the synthesis of other metal-oxide/graphene composites and this work provides a large-scale preparation route for the practical application of SnO2 in lithium ion batteries.


 Please wait while we load your content...
Please wait while we load your content...