Blue-emitting acridine-tagged silver(i)-bis-N-heterocyclic carbene†
Abstract
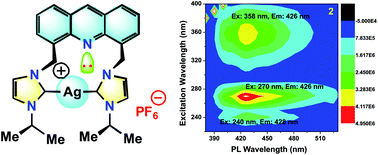
Herein, the photophysical properties of an acridine derivative of a bis-N-heterocyclic carbene silver complex were investigated. The HOMO and LUMO energy differences between 9-[(N-methyl imidazol-2-ylidene)]acridine and 4,5-bis[(N-methyl-imidazol-2-ylidene)methyl]acridine were theoretically compared. Based on the calculation, the 4,5-bis N-heterocyclic carbene-tethered acridine type of ligand was found to be a potential source for tuning the fluorescent nature of the resultant metal derivatives. Thus, a 4,5-bis N-heterocyclic carbene (NHC)-tethered acridine silver(I) salt was synthesized, and its photophysical properties were investigated. The 4,5-bis[(N-isopropylimidazol-2-ylidene)methyl]acridine silver(I) hexafluorophosphate complex was obtained from the reaction between [4,5-bis{(N-isopropylimidazolium)methyl}acridine] hexafluorophosphate and Ag2O in very good yield; this molecule was characterized by elemental analysis and FTIR, multinuclear (1H and 13C) NMR, UV-Vis, and fluorescence spectroscopic techniques. The molecular structure has been confirmed by single-crystal X-ray diffraction analysis, which has revealed that the complex is a homoleptic mononuclear silver(I) cationic solid. The charge of the Ag(I)–NHC cation is balanced by the hexafluorophosphate anion. The cationic moieties are closely packed in the chair and inverted chair forms where silver(I) possesses a quasi-linear geometry. Moreover, the silver complex provided blue emission from all the three excitations with good fluorescence quantum yield. The fluorescence lifetime of the silver(I) complex has been determined using the time-correlated single photon counting technique. Interestingly, the fluorescence decay pattern and the fluorescence lifetimes of the silver complex are largely different from those of the parent ligand acridine imidazolium salt. Moreover, the theoretical predictions have been found to be in good agreement with the experimental results.


 Please wait while we load your content...
Please wait while we load your content...