Solvent- and transition metal-free amide synthesis from phenyl esters and aryl amines†
Abstract
A general, economical, and environmentally friendly method of amide synthesis from phenyl esters and aryl amines was developed. This new method has significant advantages compared to previously reported palladium-catalyzed approaches. The reaction is performed transition metal- and solvent-free, using a cheap and environmentally benign base, NaH. This approach enabled us to obtain target amides in high yields with high atom economy.
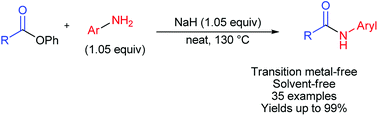


 Please wait while we load your content...
Please wait while we load your content...