A simple liquid chromatography-tandem mass spectrometry method to accurately determine the novel third-generation EGFR-TKI naquotinib with its applicability to metabolic stability assessment
Abstract
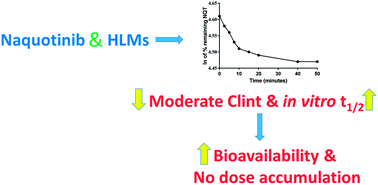
Naquotinib (ASP8273, NQT) is a novel third-generation epidermal growth factor receptor tyrosine kinase inhibitor (EGFR-TKIs). NQT was found to be more effective than osimertinib against the EGFR L858R plus T790M mutation (L858R+T790M). A rapid resolution liquid chromatography (RRLC)-tandem mass spectrometry (MS/MS) method was developed and validated for NQT quantification and its metabolic stability was investigated. NQT and foretinib (FTB) as an internal standard (IS) were separated using a mobile phase under isocratic conditions with a C18 column (reversed phase system). The linearity of the analytical method ranged from 5 to 500 ng mL−1 (coefficient of correlation [r2] ≥ 0.9999) in a human liver microsome (HLM) matrix. The limit of detection and limit of quantification were 0.78 and 2.36 ng mL−1, respectively. The inter-day and intra-day accuracy and precision were −6.36 to 1.88 and 0.99 to 2.58%, respectively. The metabolic stability of NQT in the HLM matrix was calculated using the in vitro half-life (t1/2, 67.96 min) and intrinsic clearance (Clint, 2.12 mL min−1 kg−1). NQT is considered to be a moderate extraction ratio drug that is moderately excreted from the human body compared with other related TKIs. This proposed methodology is thought to be the first method for assessing NQT concentration and its metabolic stability.


 Please wait while we load your content...
Please wait while we load your content...