Minisci C–H alkylation of N-heteroarenes with aliphatic alcohols via β-scission of alkoxy radical intermediates†
Abstract
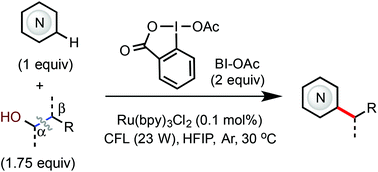
A Minisci-type C–H alkylation reaction of N-heteroarenes with aliphatic alcohols via β-scission of alkoxy radical intermediates under photoredox-catalyzed conditions was developed. The use of the benziodoxole acetate (BI-OAc) oxidant is critical to achieving high efficiency under mild conditions using a slight excess of an alcohol reactant. Primary, secondary, and tertiary alcohols all are compatible. Reactions of various complex N-heteroarenes including drug molecules and steroid natural products have been demonstrated.
- This article is part of the themed collection: 2019 Organic Chemistry Frontiers HOT articles


 Please wait while we load your content...
Please wait while we load your content...