Diels–Alder reaction on perylenediimides: synthesis and theoretical study of core-expanded diimides†‡
Abstract
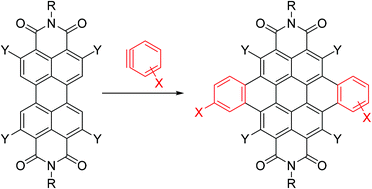
A one-step reaction for the fusion of aromatic rings to one or both bay areas of perylenediimides using benzynes is presented. Yields as high as 70% for naphthoperylenendiimide 2 and 80% for dibenzocoronenediimide 3 are obtained. The reaction is also carried out using substituted benzynes, heteroaromatic benzynes and substituted perylenediimides. A combined experimental/theoretical approach, based on measuring redox and absorption/emission properties and performing density functional theory calculations, indicates that increasing the π-skeleton of PDIs transversally leads to significant and unexpected changes in the electronic, redox and optical properties. The observed trends are rationalized in terms of molecular orbital topology and overlap according to three different levels of core expansion, and can be used as design principles for obtaining PDIs with improved functionalities.


 Please wait while we load your content...
Please wait while we load your content...