(E)-Selective Friedel–Crafts acylation of alkynes to β-chlorovinyl ketones: defying isomerizations in batch reactions by flow chemistry approaches†
Abstract
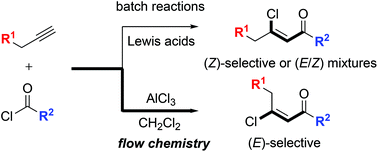
The Friedel–Crafts acylation of alkynes stereoselectively provides (Z)-β-chlorovinyl ketones, whereas the AlCl3-promoted reaction yields a mixture of stereoisomeric β-chlorovinyl ketones. To develop the (E)-selective synthesis of β-chlorovinyl ketones, a flow chemistry approach was devised for the Friedel–Crafts acylation of alkynes that defies the facile (E) → (Z) isomerization under the AlCl3-promoted reaction conditions. Compared to batch reactions, the flow chemistry approach provides a fast, clean, high yielding, and stereoselective synthetic route to (E)-β-chlorovinyl ketones.


 Please wait while we load your content...
Please wait while we load your content...