Solvent-tuned chemoselective carboazidation and diazidation of alkenes via iron catalysis†
Abstract
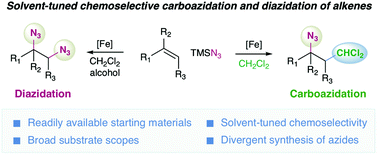
We have described Fe-catalyzed chemoselective oxidative carboazidation and diazidation of alkenes by employing CH2Cl2 as the chloromethyl source and TMSN3 as the azido source. For the synthesis, we have taken the advantage of solvent-controlled generation of C-based and N-based radicals, allowing the construction of molecularly diverse azido compounds from readily available starting materials through a simple operation.


 Please wait while we load your content...
Please wait while we load your content...