Deciphering the exceptional selectivity of semipinacol rearrangements in cis-fused β-lactam diols using high-level quantum chemical methods†
Abstract
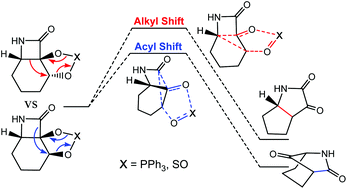
The mechanism for the semipinacol rearrangement in cis-fused β-lactam diols has been examined using highly accurate double-hybrid density functional theory methods. This reaction involves a competition between two possible migrating groups (alkyl and acyl), which can undergo a 1,2 C–C bond migration. We find that acyl migration is both kinetically and thermodynamically more favorable. These results are consistent with experimental observations and are rationalized based on conformational, structural, and orbital interaction analysis. We proceed to investigate the semipinacol rearrangement in trans-fused β-lactam diol and propose that this system undergoes a reversed selectivity which favors the alkyl migration.


 Please wait while we load your content...
Please wait while we load your content...