Influence of ions to modulate hydrazone and oxime reaction kinetics to obtain dynamically cross-linked hyaluronic acid hydrogels†
Abstract
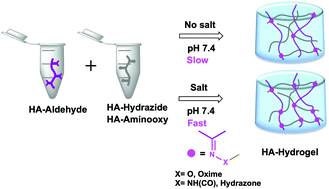
Dynamic covalent chemistry forming hydrazone and oxime linkages is attractive due to its simplicity, selectivity and compatibility under aqueous conditions. However, the low reaction rate at physiological pH hampers its use in biomedical applications. Herein, we present different monovalent and bivalent aqueous salt solutions as bio-friendly, non-toxic catalysts which can drive the hydrazone and oxime reactions with excellent efficacy at physiological pH. Direct comparison of hydrazone and oxime reactions using a small molecule model, without any salt catalysis, indicated that oxime formation is 6-times faster than hydrazone formation. Addition of different salts (NaCl, NaBr, KCl, LiCl, LiClO4, Na2SO4, MgCl2 and CaCl2) accelerated the pseudo-first-order reaction kinetics by ∼1.2–4.9-fold for acylhydrazone formation and by ∼1.5–6.9-fold for oxime formation, in a concentration-dependent manner. We further explored the potential of such catalysts to develop acylhydrazone and oxime cross-linked hyaluronic acid (HA) hydrogels with different physicochemical properties without changing the degree of chemical modification. Analogous to the small molecule model system, the addition of monovalent and divalent salts as catalysts significantly reduced the gelling time. The gelling time for the acylhydrazone cross-linked HA-hydrogel (1.6 wt%) could be reduced from 300 min to 1.2 min by adding 100 mM CaCl2, while that for the oxime cross-linked HA-hydrogel (1.2 wt%) could be reduced from 68 min to 1.1 min by adding 50 mM CaCl2. This difference in the gelling time also resulted in hydrogels with differential swelling properties as measured after 24 h. Our results are the first to demonstrate the use of salts, for catalyzing hydrogel formation under physiologically relevant conditions.


 Please wait while we load your content...
Please wait while we load your content...