Cationic copolymerization of isosorbide towards value-added poly(vinyl ethers)†
Abstract
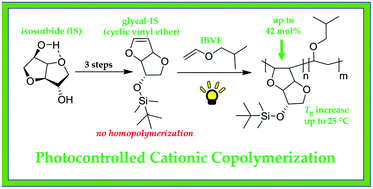
Biomass-derived isosorbide (IS) was converted into a mono-glycal (i.e. vinyl ether) derivative (Gly-IS) to investigate its efficacy for cationic polymerization. While homopolymerization was unsuccessful, likely due to the steric demand near the propagating cationic site, copolymerization with isobutyl vinyl ether (IBVE) revealed great promise for the use of Gly-IS as a rigid and sustainable comonomer. Traditional cationic methods yielded copolymers with IBVE, but the incorporation of Gly-IS was hindered by the propensity for Lewis acids to catalyze a ring-opening reaction driven by aromatization to a chiral furan analog. This reaction was discovered to be significantly sequestered through the use of metal-free photoinitiated cationic copolymerization methods that are void of Lewis acid reagents, yielding a much higher incorporation of Gly-IS (up to 42 mol%) into the copolymer. The rigidity and chirality of the Gly-IS repeating unit was found to increase the glass transition temperature (Tg) up to 25 °C with 33 mol% incorporation at modest molar mass (10.4 kg mol−1) while all copolymers displayed thermal stability up to 320 °C under inert atmosphere. Due to its chiral structure, specific optical rotation [α] of the copolymer also increased with incorporation of Gly-IS. Therefore, Gly-IS presents opportunity as a sustainable and value-added comonomer to modulate the properties of common poly(vinyl ether) systems.


 Please wait while we load your content...
Please wait while we load your content...