Physical gelation of AB-alternating copolymers made of vinyl phenol and maleimide units: cooperation between precisely incorporated phenol and long alkyl pendant groups†
Abstract
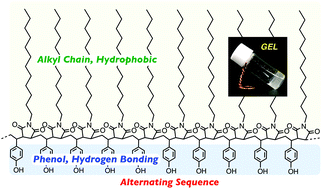
A series of alternating copolymers consisting of vinyl phenol and n-alkyl maleimide was synthesized via radical copolymerization of a protected styrene derivative with a functional maleimide monomer followed by deprotection. Copolymers carrying long alkyl pendants such as C12H25– or C18H37– chains on the maleimide unit showed a UCST-type thermal response in aromatic solvents, and organogels were specifically formed upon cooling of the fluid solution prepared at higher temperatures. Hydrogen bonding of the phenol units is crucial for gelation, and the gelation temperature and stiffness were tunable by varying the concentration, solvent and polymerization degree. Analyses by 1H-NMR, linear rheology, WAXD, SANS and cryo-TEM gave the picture of vermicular self-assembled nano-objects formed through segregated and hydrogen-bonded packing by the precisely incorporated two units in an alternating sequence.


 Please wait while we load your content...
Please wait while we load your content...