Synthesis of water-soluble hypervalent iodine reagents for fluoroalkylation of biological thiols†
Abstract
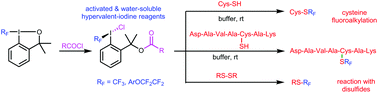
New open-chain and water-soluble hypervalent iodine reagents were synthesized and used for the transfer of fluoroalkyl groups to sulfur atoms of cysteine and cysteine-containing peptides under biocompatible conditions. Some of the reagents displayed excellent reactivity despite their limited stability in aqueous media. In reactions with a short cysteine-containing peptide, in addition to the expected S-fluoroalkylated product, a range of side-products were obtained. The amount of side-products depended on the conditions used (type of reagent, concentration, and pH). With highly activated hypervalent iodine reagents, a new reactive mode was observed – reaction with disulfides to form fluoroalkyl thiols.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...