Synthesis and application of light-switchable arylazopyrazole rapamycin analogs†
Abstract
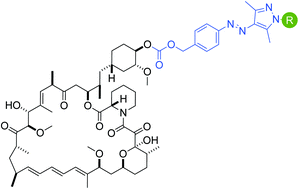
Rapamycin-induced dimerization of FKBP and FRB has been utilized as a tool for co-localizing two proteins of interest in numerous applications. Due to the tight binding interaction of rapamycin with FKBP and FRB, the ternary complex formation is essentially irreversible. Since biological processes occur in a highly dynamic fashion with cycles of protein association and dissociation to generate a cellular response, it is useful to have chemical tools that function in a similar manner. We have developed arylazopyrazole-modified rapamycin analogs which undergo a configurational change upon light exposure and we observed enhanced ternary complex formation for the cis-isomer over the trans-isomer for one of the analogs.
- This article is part of the themed collection: Chemical Biology in OBC


 Please wait while we load your content...
Please wait while we load your content...