α-Halogenoacetamides: versatile and efficient tools for the synthesis of complex aza-heterocycles
Abstract
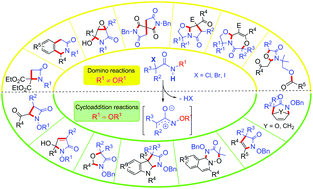
This review provides an overview of the applications of α-halogenoacetamides in domino and cycloaddition reactions. α-Halogenoacetamides are versatile building blocks that can lead to a wide variety of complex aza-heterocycles of biological interest when engaged in domino and/or cycloaddition reactions. The reactivity and the reaction conditions involved for these species (solvent, base, etc.) are closely related to the substituent onto the nitrogen atom of the amide: N-alkyl α-halogenoacetamides usually act as formal 1,3-dipoles in domino processes whereas N-alkoxy derivatives often react as real 1,3-dipoles via the formation of aza-oxyallyl cation species. This important modulation of the reactivity of these compounds opens the way to a large panel of reactions and therefore to a large diversity of aza-heterocycles.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...