Synthesis of glycosyl chlorides using catalytic Appel conditions†
Abstract
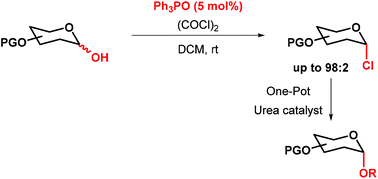
The stereoselective synthesis of glycosyl chlorides using catalytic Appel conditions is described. Good yields of α-glycosyl chlorides were obtained using a range of glycosyl hemiacetals, oxalyl chloride and 5 mol% Ph3PO. For 2-deoxysugars treatment of the corresponding hemiacetals with oxalyl chloride without phosphine oxide catalyst also gave good yields of glycosyl chloride. The method is operationaly simple and the 5 mol% phosphine oxide by-product can be removed easily. Alternatively a one-pot, multi-catalyst glycosylation can be carried out to transform the glycosyl hemiacetal directly to a glycoside.
- This article is part of the themed collections: Chemical Biology in OBC and Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...