Asymmetric total synthesis of paecilomycin C through intramolecular nucleophilic ring opening of an epoxide†
Abstract
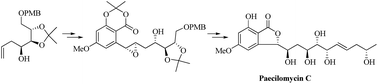
The convergent total synthesis of naturally occurring paecilomycin C is described here for the first time. Asymmetric Brown allylation, E-selective cross metathesis, and a biomimetic carboxylate assisted intramolecular nucleophilic ring opening of an epoxide were employed to access the enantiopure γ-lactone framework of the natural product. Late stage E-selective Julia–Kocienski olefination was then employed to furnish the natural product in an efficient way.
- This article is part of the themed collection: Total synthesis in OBC


 Please wait while we load your content...
Please wait while we load your content...