Protein labelling and albumin binding characteristics of the near-IR Cy7 fluorophore, QuatCy†
Abstract
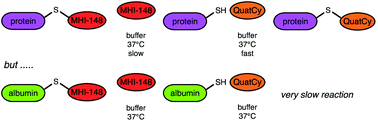
Free cysteine residues react with QuatCy 1, by simply mixing the protein and dye in aqueous buffer at 37 °C. Another dye, MHI-148, can be used for a similar labelling protocol, but QuatCy reacts faster with all proteins studied, except albumin; it emerges here that this is because MHI-148 instantly forms of a non-covalent complex with albumin, but QuatCy does not. Labelling with QuatCy has advantages insofar as it is over five times brighter, and much more photostable, than MHI-148, and combination labelling with this dye pair will allow multiplexing in the near-IR region.


 Please wait while we load your content...
Please wait while we load your content...
