Melanin production by tyrosinase activity on a tyrosine-rich peptide fragment and pH-dependent self-assembly of its lipidated analogue†
Abstract
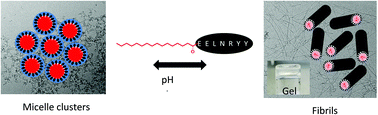
We investigate the self-assembly of a palmitoylated (C16-chain at the N terminus) peptide fragment in comparison to the unlipidated peptide EELNRYY, a fragment of the gut hormone peptide PYY3–36. The lipopeptide C16-EELNRYY shows remarkable pH-dependent self-assembly above measured critical aggregation concentrations, forming fibrils at pH 7, but micelles at pH 10. The parent peptide does not show self-assembly behaviour. The lipopeptide forms hydrogels at sufficiently high concentration at pH 7, the dynamic mechanical properties of which were measured. We also show that the tyrosine functionality at the C terminus of EELNRYY can be used to enzymatically produce the pigment melanin. The enzyme tyrosinase oxidises tyrosine into 3,4-dihydroxyphenylalanine (DOPA), DOPA-quinone and further products, eventually forming eumelanin. This is a mechanism of photo-protection in the skin, for this reason controlling tyrosinase activity is a major target for skin care applications and EELNRYY has potential to be developed for such uses.


 Please wait while we load your content...
Please wait while we load your content...