Phosphine-catalyzed regiospecific (3 + 2) cyclization of 3-nitroindoles with allene esters†
Abstract
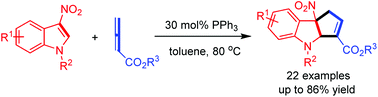
A phosphine-catalyzed regiospecific (3 + 2) cyclization of 3-nitroindoles with allene esters has been established, which constructed indole-fused five-membered rings in good yields (up to 86%). This reaction serves as a powerful method for the construction of indole-fused five-membered rings. In addition, this reaction provides a useful strategy toward settling the challenge of utilizing other 1,3-dipoles or 1,3-dipole surrogates to perform (3 + 2) cyclizations with 3-nitroindoles in different catalytic modes, which will enrich the chemistry of nitroindoles and catalytic asymmetric dearomatization cyclizations.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...