Hydroaminoalkylation of sterically hindered alkenes with N,N-dimethyl anilines using a scandium catalyst†
Abstract
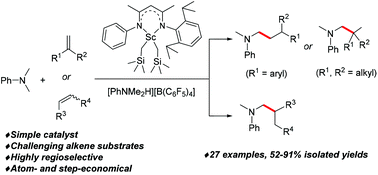
An atom- and step-economical C(sp3)–H addition of N,N-dimethyl anilines to various sterically demanding 1,1- and 1,2-disubstituted alkenes has been achieved by using a simple β-diketiminato ligand supported scandium dialkyl complex in combination with a borate compound. The corresponding C(sp3)–C(sp3) bond forming reaction occurs with excellent regioselectivities to give a variety of tertiary aromatic amines.
- This article is part of the themed collections: Synthetic methodology in OBC and New Talent


 Please wait while we load your content...
Please wait while we load your content...