Access to 3-spiroindolizines containing an isoindole ring through intra-molecular arylation of spiro-N-acyliminium species: a new family of potent farnesyltransferase inhibitors†
Abstract
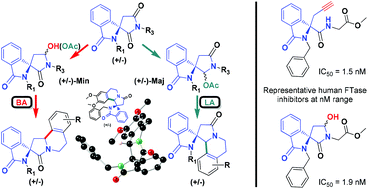
Based on N-acyliminium species, two efficient and rapid approaches to diversify spirocyclic systems connected by two different carbon centers to the isoindole ring have been developed. The imide reduction and the tandem oxidative cleavage of olefin/formyl-amide equilibration were at first selected as the key steps for these strategies. Ultimately the intramolecular α-amidoalkylation reaction was achieved through the arylation of α-acetoxy lactams or α-hydroxy lactams using, respectively, a Lewis acid or a Brønsted acid depending on the nature of N-acyliminium precursors. The latter led, in addition to the spiro-6-membered aza-heterocycles, to the formation of scarce spiro-5-membered analogues which show promising inhibitory activities on human farnesyltransferase in the nanomolar range demonstrating improved IC50 values of up to 1.5 nM.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...