Ambient synthesis of nanomaterials by in situ heterogeneous metal/ligand reactions†
Abstract
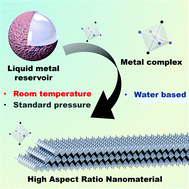
Coordination polymers are ideal synthons in creating high aspect ratio nanostructures, however, conventional synthetic methods are often restricted to batch-wise and costly processes. Herein, we demonstrate a non-traditional, frugal approach to synthesize 1D coordination polymers by in situ etching of zerovalent metal particle precursors. This procedure is denoted as the heterogeneous metal/ligand reaction and was demonstrated on Group 13 metals as a proof of concept. Simple carboxylic acids supply the etchant protons and ligands for metal ions (conjugate base) in a 1 : 1 ratio. This scalable reaction produces a 1D polymer that assembles into high-aspect ratio ‘nanobeams’. We demonstrate control over crystal structure and morphology by tuning the: (i) metal center, (ii) stoichiometry and (iii) structure of the ligands. This work presents a general scalable method for continuous, heat free and water-based coordination polymer synthesis.


 Please wait while we load your content...
Please wait while we load your content...