Bromine polycondensation in pristine and fluorinated graphitic carbons†
Abstract
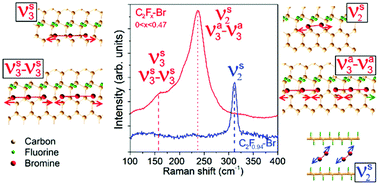
Despite decades of study the precise behavior of bromine in graphitic carbons remains unclear. In this report, using Raman spectroscopy, we reveal two types of bromine structure in graphitic carbon materials. Between fluorinated graphene layers with a composition close to C2F, Br2 molecules are intercalated in a form similar to liquid bromine. Bromination of pristine and low-fluorinated graphitic carbons behaves very differently with distinct Br-related Raman spectra. With the guidance of density functional theory (DFT) calculations, all Raman features are assigned to normal vibration modes of specific bromine species over graphene and fluorinated graphene. When intercalated between extended non-fluorinated sp2-hybridized carbon regions, physisorbed Br2 molecules move freely across the non-functionalized region toward the CF border. Multiple Br2 molecules then combine spontaneously into Br3-based chains, whose coupling activates otherwise Raman inactive modes. Significant charge transfer to bromine species occurs in this case. DFT calculated frequencies match precisely the experimental Br-related Raman bands observed in the intercalation carbon compounds. The fluorine-catalyzed bromine chain-formation process shown here is general and should also operate with edges and other defect species.


 Please wait while we load your content...
Please wait while we load your content...