Film morphology of acrylonitrile materials deposited by a solution process and vacuum evaporation. Supramolecular interactions, optoelectronic properties and an approximation by computational calculations†
Abstract
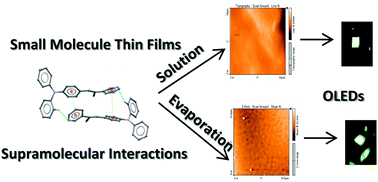
α,β-Unsaturated acrylonitrile compounds with a pyridine scaffold are fluorescent materials with efficient solid-state emission and electroluminescence properties. In this work, three low molecular weight compounds, i.e., small molecules, with the donor group 4-(diphenylamino)phenyl (D) and a pyridine-substituent as an acceptor (A) attached to α,β-unsaturated nitrile and one with phenyl were used as the emissive layer in OLEDs. The emissive layer was deposited by either a solution or high vacuum evaporation process to provide insight into the effect of morphology differences on the OLED performance. Notably, for the solution process the better morphology was achieved with dioxane. For devices two cathodes were used, evaporated aluminum and the eutectic alloy Field's metal composed of Bi, In and Sn, which was deposited by drop casting. Independent of the used cathode and the technique for its deposition, the device performance for both cathodes was similar. Electrical properties such as the threshold voltage, about 3–8 volts, and the current density were affected by the chemical structure of the emitting material, but not by the metal used as a cathode. Results showed that the eutectic alloy is a good alternative as a cathode in organic light emitting diodes. Also, we reported the quantitative analysis of intermolecular interactions by PIXEL and density functional theory (DFT) calculations.


 Please wait while we load your content...
Please wait while we load your content...