Catalytic synthesis of benzimidazoles and organic carbamates using a polymer supported zinc catalyst through CO2 fixation†
Abstract
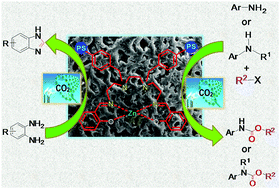
Utilization of carbon dioxide in chemical fixation for synthesis of fine chemicals like benzimidazoles, organic carbamates, etc. is in high demand in recent years as carbon dioxide is a cost effective, sustainable, and green renewable C1 source. In this article we present the design and synthesis of an organically modified polystyrene bound heterogeneous [PS-Zn(II)-SALTETA] catalyst. The catalyst has been characterized thoroughly by Fourier transform infrared spectroscopy, atomic absorption spectroscopy, thermo gravimetric analysis, PXRD, SEM and EDAX studies. The catalyst was used for cyclization of o-phenylenediamines through insertion of carbon dioxide in order to produce benzimidazoles in the presence of dimethylamine borane (DMAB). The developed catalytic procedure is sustainable, economical and efficient owing to the utilization of ethanol/water as a biodegradable and environment friendly solvent system. Besides benzimidazole production the catalyst was also very active for manufacture of organic carbamates from anilines and n-butyl bromide under atmospheric CO2 pressure under solvent free conditions at room temperature and the catalytic protocol showed outstanding functional group tolerance. Moreover the catalyst is highly recyclable and reusable.


 Please wait while we load your content...
Please wait while we load your content...