Influence of the channel size of isostructural 3d–4f MOFs on the catalytic aerobic oxidation of cycloalkenes†
Abstract
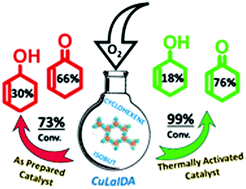
The present work reports a new group of heterogeneous catalysts with a 3D structure, CuLnIDA, {[Cu3Ln2(IDA)6]·8H2O} (Ln: LaIII, GdIII or YbIII), with an organic linker (H2IDA: iminodiacetic acid). Different sets of O2 pressure and time were used in order to obtain the optimal reaction conditions at 75 °C. The reaction was found to depend on the [aldehyde]/[substrate] ratio. The best results, with a conversion of 73% for CuLaIDA as the catalyst, were obtained for the smallest ratio of 0.2. Finally, the importance of the pore size was analysed by comparing the catalytic activity of the as formed catalyst with that of the thermally activated one. The conversion increased ca. 26–35% for the different catalysts when they were previously activated. In addition, the selectivity increased towards cyclohexenone. The use of molecular oxygen as the oxidizing agent in a system where an auxiliary solvent is not used, as the cyclohexene substrate and products play the role of a solvent, permitted us to generate a more friendly environmental system for the oxidation of cycloalkenes under mild conditions.


 Please wait while we load your content...
Please wait while we load your content...