The effect of the electrophilic group on the hierarchy of nucleofuges in the aminolysis reactions of thiol- and dithiocarbonates with secondary alicyclic amines: A kinetic and theoretical study†
Abstract
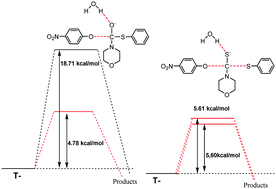
The aminolysis reactions of O-(4-nitrophenyl) S-aryl dithiocarbonates with different secondary alicyclic amines (SAA) lead to the formation of different 4-nitrophenyl : S-aryl ratios (56 : 44 and 47 : 53 for benzenethiol and 4-chlorobenzenethiol, respectively). For the corresponding thiolcarbonates, 4-nitrophenol was the lone product, evidencing the effect of the electrophile moiety on the nucleofugacity hierarchy. The kinetic results show that in the mechanism there are two tetrahedral intermediates: one zwitterionic (T±) and one anionic (T−). As model reactions, the reactions of O-(4-nitrophenyl) S-phenyl thiol- and dithiocarbonates with morpholine were theoretically examined using DFT methods. Theoretical calculations were performed using a continuous solvation model in the absence and presence of one and two explicit water molecules. The microsolvation of T− by one explicit water molecule was predicted as having O-(4-nitrophenyl) : S-phenyl distributions of 52 : 48 and 98 : 2 for dithiocarbonates and thiolcarbonates, respectively. These results have allowed us to establish that processes such as microsolvation, electronic distribution and the structure of the reactive centers participate in the departure of the leaving groups in the thiolcarbonate and dithiocarbonate aminolysis, although the preferential solvation of these centers seems to have a predominant role in the nucleofuge hierarchy.


 Please wait while we load your content...
Please wait while we load your content...