Ion-pair complexes of Schiff base Fe(iii) cations and complex anions†
Abstract
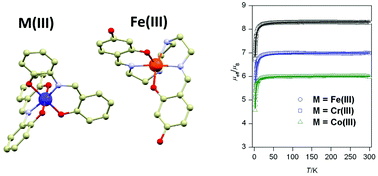
Compound [Fe(H2-4OH-L6)]Cl was used for preparation of four new ion-pair complexes with general formula: [Fe(H2-4OH-L6)][M(L3)2]·H2O (M = CoIII (3a), CrIII (3b) and FeIII (3c)) and [Fe(H2-4OH-L6)][Ag(CN)2] (3d), where H4-4OH-L6 = N,N′-bis[2,4-dihydroxy-(benzylideneamino)ethyl]ethane-1,2-diamine and H2L3 = 2-{(E)-[(2-hydroxyphenyl)imino]methyl}phenol. Furthermore, two [Fe(H-4OH-L6)] complexes (1a and 1b) with monodeprotonated hexadentate ligands were prepared. The crystal structures were determined by single-crystal X-ray measurements for all the above-mentioned complexes excluding 3c. Its isostructurality with 3a–b was confirmed by powder X-ray diffraction. The magnetic properties were investigated by static magnetic measurements and they are dominated by the high spin ground state of the complex cations and anions and at a smaller scale by the intermolecular interactions present among the molecules. The susceptibility and magnetization data were fitted simultaneously using the model for monomeric complexes including the zero-field splitting and the molecular field correction and these studies were supported also by CASSCF/NEVPT2 and BS-DFT calculations.


 Please wait while we load your content...
Please wait while we load your content...