Construction of a 3D porous Co(ii) metal–organic framework (MOF) with Lewis acidic metal sites exhibiting selective CO2 capture and conversion under mild conditions†
Abstract
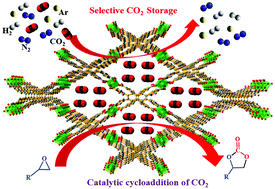
A porous, 3D Co(II) metal–organic framework, [{Co(TCPB)0.5(H2O)}·DMF]n (MOF1), (where H4TCPB = 1,2,4,5-tetrakis(4-carboxyphenyl)benzene) constituted by a rigid, tetratopic, H4TCPB ligand was synthesized under solvothermal conditions. Single crystal X-ray structure determination revealed the 3D framework structure of MOF1 with 1D channels of dimensions 10.5 × 10.5 Å2 decorated with Co(II) ions in the crystallographic a-axis. Gas adsorption studies showed the selective adsorption property of MOF1 for CO2 over other (N2, Ar and H2) gases with an isosteric heat of adsorption (Qst) value of 35.4 kJ mol−1. The considerably high value of Qst has been ascribed to the stronger interaction of CO2 molecules with the Co(II) metal sites lined in the 1D channels of MOF1. Interestingly, the coordinated water molecules at the Co(II) centers can be removed by activating MOF1 at a temperature of 120 °C to generate a framework with pores lined with unsaturated Lewis acidic Co(II) ions. The activated MOF1 shows a very good catalytic activity for highly selective, solvent-free, heterogeneous conversion of CO2 into cyclic carbonates under mild conditions of an atmospheric pressure of CO2. Further, the MOF1 catalyst was facilely recycled and reused for several cycles without substantial loss of catalytic activity.


 Please wait while we load your content...
Please wait while we load your content...