Di- and tetramethoxy benzothienobenzothiophenes: substitution position effects on the intermolecular interactions, crystal packing and transistor properties†
Abstract
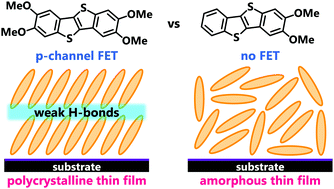
We report the synthesis, structures, and transistor properties of benzothienobenzothiophene (BTBT) derivatives tailored by introducing methoxy groups at the 2,3- and 2,3,7,8-positions (2,3-dimethoxy-BTBT: BTBT(OMe)2 and 2,3,7,8-tetramethoxy-BTBT: BTBT(OMe)4). Both of these compounds form a herringbone packing in the crystal; however, their molecular arrangements are significantly different: the tetramethoxy derivative BTBT(OMe)4 shows no dimerization, whereas the dimethoxy derivative BTBT(OMe)2 shows significant tetramerization with two dimers. This difference, attributable to the difference in the substitution pattern of the methoxy groups or the molecular symmetry, leads to a drastic difference in their charge transport properties. In the vapor-deposited thin films, BTBT(OMe)4 exhibits a crystalline architecture similar to the bulk phase stabilized by weak hydrogen bonds, showing good p-channel transistor performance, while BTBT(OMe)2 exhibits an amorphous-like structure without any observable transport properties.


 Please wait while we load your content...
Please wait while we load your content...