Nanostructure of the deep eutectic solvent/platinum electrode interface as a function of potential and water content†
Abstract
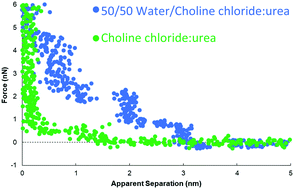
The interfacial nanostructure of the three most widely-studied Deep Eutectic Solvents (DESs), choline chloride:urea (ChCl:Urea), choline chloride:ethylene glycol (ChCl:EG), and choline chloride:glycerol (ChCl:Gly) at a Pt(111) electrode has been studied as a function of applied potential and water content up to 50 wt%. Contact mode atomic force microscope (AFM) force–distance curves reveal that for all three DESs, addition of water increases the interfacial nanostructure up to ∼40 wt%, after which it decreases. This differs starkly from ionic liquids, where addition of small amounts of water rapidly decreases the interfacial nanostructure. For the pure DESs, only one interfacial layer is measured at OCP at 0.5 nm, which increases to 3 to 6 layers extending ∼5 nm from the surface at 40 or 50 wt% water. Application of a potential of ±0.25 V to the Pt electrode for the pure DESs increases the number of near surface layers to 3. However, when water is present the applied potential attenuates the steps in the force curve, which are replaced by a short-range exponential decay. This change was most pronounced for ChCl:EG with 30 wt% or 50 wt% water, so this system was probed using cyclic voltammetry, which confirms the interfacial nanostructure is akin to a salt solution.


 Please wait while we load your content...
Please wait while we load your content...