MOF-templated cobalt nanoparticles embedded in nitrogen-doped porous carbon: a bifunctional electrocatalyst for overall water splitting†
Abstract
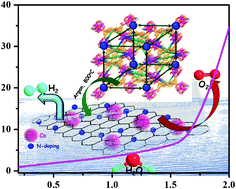
Development of cost-effective and efficient non noble metal electrocatalysts has immense importance towards sustainable energy technologies. Herein, a newly constructed porous Co(II)-metal organic framework (MOF) has been utilized for the synthesis of cobalt nanoparticles embedded in N-doped porous carbon, (Co@NPC), via a facile MOF-annealing strategy, at an optimum temperature of 800 °C under an argon atmosphere. DMF molecules present in the form of solvated guests and cations within the 3D-framework serve as a source for N-doping during the formation of the porous graphitic carbon upon carbonization. The nanocomposite was found to encapsulate homogeneously dispersed cobalt nanoparticles within the N-doped porous carbonaceous matrix. The synergistic effect of cobalt nanoparticles and the heteroatom-doped carbon framework makes Co@NPC electrochemically active towards both the oxygen evolution reaction (OER) and hydrogen evolution reaction (HER) under alkaline conditions. Furthermore, Co@NPC exhibits outstanding performance as a bifunctional electrocatalyst towards electrochemical water splitting with remarkable stability and durability. It achieves a current density of 10 mA cm−2 at a low cell voltage of 1.66 V in 1 M NaOH solution which is comparable with that of most of the self-templated ZIF-derived non-noble metal electrocatalysts.


 Please wait while we load your content...
Please wait while we load your content...