Highly sensitive and room temperature detection of ultra-low concentrations of O3 using self-powered sensing elements of Cu2O nanocubes
Abstract
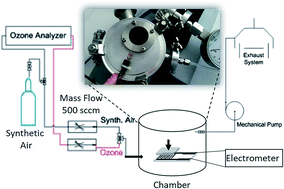
The fundamental development of the design of novel self-powered ozone sensing elements, operating at room temperature, based on p-type metal oxides paves the way to a new class of low cost, highly promising gas sensing devices. In this work, p-type Cu2O nanocubes were synthesized by a simple solution-based method and tested as a self-powered ozone sensing element, at room temperature (25 °C) for the first time. Highly crystalline Cu2O nanocubes with 30 nm size were characterized by X-ray Diffraction (XRD), Scanning Electron Microscopy (SEM) and Transmission Electron Microscopy (TEM). Self-powered sensing elements of Cu2O nanocubes have been successfully fabricated by deposition of Cu2O nanocubes on interdigitated electrodes (IDEs) consisting of two connection tracks with 500 digits and a gap of 5 μm in order to investigate their response to ozone at room temperature. The experimental results showed that the use of nanocubes as sensing elements was suitable for detecting ultra-low concentrations of O3 down to 10 ppb at room temperature with very high sensitivity (28%) and a very low response/recovery time. The reversible sensing process of the relatively weak binding of O3 species by trapping sites on Cu2O facets with increased oxygen content was studied by using density functional theory (DFT) calculations.
- This article is part of the themed collection: Gas sensing


 Please wait while we load your content...
Please wait while we load your content...