Asymmetric bifunctional protein nanoparticles through redesign of self-assembly†
Abstract
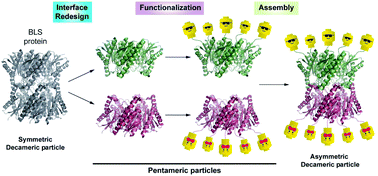
Engineering oligomeric protein self-assembly is an attractive approach to fabricate nanostructures with well-defined geometries, stoichiometry and functions. The homodecamer Brucella Lumazine Synthase (BLS) is a highly stable and immunogenic protein nanoparticle (PNP). Here, we engineered the BLS protein scaffold to display two functions in spatially opposite regions of its structure yielding a Janus-like nanoparticle. An in silico analysis of the BLS head-to-head dimer of homopentamers shows major inter-pentameric interactions located in the equatorial interface. Based on this analysis, two BLS protomer variants were designed to interrupt pentamer self-dimerization and promote heteropentameric dimers. This strategy enabled us to generate a decameric particle with two distinct sides formed by two independent pentamers. The versatility of this new self-assembly nanofabrication strategy is illustrated with two example applications. First, a bifunctional BLS bearing Alexa Fluor 488 fluorophores on one side and sialic acid binding domains on the other side was used for labelling murine and human cells and analyzed by flow cytometry and confocal microscopy. Second, multichromophoric FRET nanoparticles were fabricated and characterized at the single molecule level, showing discrete energy transfer events. The engineered BLS variants constitute a general platform for displaying two functions in a controlled manner within the same PNP with potential applications in various areas such as biomedicine, biotechnology and nanotechnology.


 Please wait while we load your content...
Please wait while we load your content...