Single-molecule nanoscale drug carriers with quantitative supramolecular loading†
Abstract
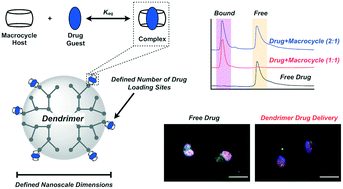
While there are examples of successful nanomedicine technologies, challenges remain in engineering systems with controlled and defined characteristics such as size and drug loading. Dendrimers are a class of synthetic macromolecules which offer routes to precise nanoscale objects. Here, highly efficient and orthogonal methods for dendrimer synthesis are combined with the covalent attachment of a tunable number of cucurbit[7]uril macrocycle carriers. This synthetic macrocycle affords uniquely high-affinity binding to certain guests, lending modularity to the prepared dendrimer in terms of its drug cargo. To verify this concept, the chemotherapeutic doxorubicin was conjugated to a high-affinity guest for CB[7]. When combined with CB[7]-modified dendrimers, quantitative drug loading was realized, with one drug bound per CB[7] on the dendrimer. The guest-modified drug was attached via a pH-labile linker to facilitate release at sites of disease or upon cell internalization. This approach offers a new combination of discrete and efficient synthetic methodology coupled with modular and high-affinity supramolecular motifs to engineer platforms with exceptional precision for use in drug delivery.
- This article is part of the themed collection: MSDE Emerging Investigators 2020


 Please wait while we load your content...
Please wait while we load your content...