Expansion of the structure–activity relationships of BACE1 inhibitors by harnessing diverse building blocks prepared using a unified synthetic approach†
Abstract
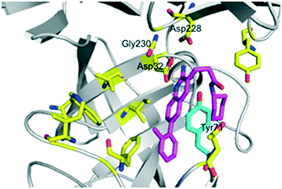
The structural diversity of β-site amyloid precursor protein cleaving enzyme 1 (BACE1) inhibitors was expanded by harnessing diverse building blocks that had been prepared via a unified lead-oriented synthetic approach. It was shown that the lipophilic cyclohexylmethyl group within a known series of BACE1 inhibitors could be productively replaced with a range of alternative ring systems.


 Please wait while we load your content...
Please wait while we load your content...