Biocatalytic retrosynthesis approaches to d-(2,4,5-trifluorophenyl)alanine, key precursor of the antidiabetic sitagliptin†
Abstract
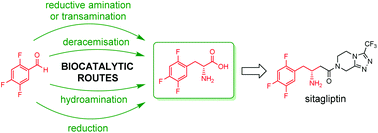
The integration of biocatalytic steps in retrosynthetic analysis of a target molecule offers multiple advantages, such as reduction of the environmental footprint of the process, viability of milder and safer reaction conditions, and accessibility of transformations that are challenging with traditional chemical synthesis. Herein, six chemo-enzymatic routes are described for the synthesis of a fluorinated D-phenylalanine derivative, precursor of the blockbuster antidiabetic drug sitagliptin. All routes start from the same aldehyde precursor and involve at least one biocatalytic step, including reductive amination, transamination, deracemisation, hydroamination, and alkene reduction. The target molecule was obtained in 2–5 steps from the aldehyde, with ee up to >99% and in 36–62% isolated yield. Furthermore, as part of one of the routes, the first example of a fully biocatalytic conversion of a cinnamic acid derivative to the corresponding D-phenylalanine (formal D-selective hydroamination) is reported.
- This article is part of the themed collection: 2019 Green Chemistry Hot Articles


 Please wait while we load your content...
Please wait while we load your content...