Synthesis of 2-substituted indoles through cyclization and demethylation of 2-alkynyldimethylanilines by ethanol†
Abstract
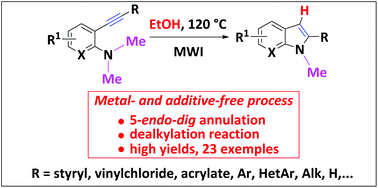
Herein, we demonstrated that 2-alkynyldimethylamines easily cyclize in EtOH according to a 5-endo-dig annulation into 2-substituted indoles without the aid of any additives or any metal catalysts to activate the triple bond. Thus, a variety of functionalized 2-styrylindoles, 2-arylindoles, 2-alkynylindoles, and 2-alkylindoles were prepared in high to excellent yields according to an environmentally friendly protocol. The mechanism has been explored to better understand this eco-friendly access to 2-substituted indoles and DFT calculations rationalized the role of the solvent in this N-annulation/dealkylation process.


 Please wait while we load your content...
Please wait while we load your content...