A tunable precious metal-free system for selective oxidative esterification of biobased 5-(hydroxymethyl)furfural†
Abstract
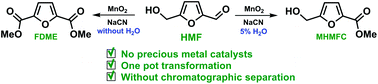
Oxidative esterification of biomass-derived 5-(hydroxymethyl) furfural (HMF) and furfural and their derivatives has been performed using a simple MnO2/NaCN system. The developed method allows the selective one-pot transformation of HMF to dimethyl furan-2,5-dicarboxylate (FDME) in 83% isolated yield without the formation of a free acid. Simplification of FDME production provides the missing link for manufacturing sustainable value-added materials from biomass. Addition of water to the oxidative system allows fine-tuning of reaction selectivity to obtain the previously difficult-to-access pure methyl 5-(hydroxylmethyl)furan-2-carboxylate in one step directly from the unprotected HMF without chromatographic separation.


 Please wait while we load your content...
Please wait while we load your content...