Recovery and conversion of acetic acid from a phosphonium phosphinate ionic liquid to enable valorization of fermented wastewater†
Abstract
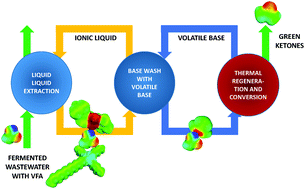
Production of volatile fatty acids (VFAs) by fermentation is a potential sustainable alternative for conventional petrochemical routes to VFAs. Due to the low VFA content of fermentation broths, robust and economical separation technology has to be devised to recover the VFA. Liquid–liquid extraction of VFAs with the phosphonium phosphinate ionic liquid (IL) [P666,14][Phos] allows good VFA extractability. For an extraction process using [P666,14][Phos] to be green, it is essential to efficiently regenerate the solvent and recover the VFA. To obtain insight into the (strong) intermolecular interactions between [P666,14][Phos] and acetic acid, selected as a model VFA, 1H NMR, 31P NMR, FT-IR and isothermal titration calorimetry (ITC) were applied. The observations were used to interpret operations to recover acetic acid from the IL, which included evaporation at elevated temperature under vacuum, possibly assisted by nitrogen stripping, in situ esterification and back-extraction with volatile bases. Through evaporative regeneration with nitrogen stripping, HAc could be removed, but only down to an HAc/IL molar ratio of 1. The remaining molar equivalent of HAc–IL interacts tightly with the IL by partial proton transfer and strong hydrogen bonding interactions with the phosphinate anion. Back-extraction of HAc with trimethylamine (TMA) and subsequent decomposition of the HAc–TMA complexes allowed for successful IL regeneration. This process uses ten times less amine (TMA) than conventional amine-based extraction processes (e.g. tri-n-octyl amine), and provides a sustainable process route to obtain pure carboxylic acids from highly diluted aqueous solutions without generating large streams of byproducts. Further valorization via in-line vaporization/catalytic ketonization or via in-line thermal decomposition and ketonization of the TMA–HAc salt was also demonstrated, showing the potential of the VFAs as a green platform for bio-based chemicals.


 Please wait while we load your content...
Please wait while we load your content...