A quantitative one-pot synthesis method for industrial azo pigments with recyclable wastewater†
Abstract
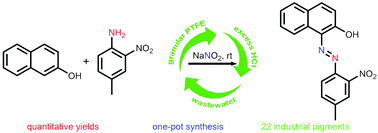
Most industrial azo pigments are synthesized by diazotization in one pot and then coupling in another. This two-pot process has been transformed into a one-pot method by adding granular PTFE (polytetrafluoroethylene) to a mechanically agitated aqueous mixture of NaNO2, HCl, a diazo component and a coupling component. This method avoids the step of using a base or a surfactant to dissolve the coupling component. The reactions were fast and quantitative. The granular PTFE, wastewater and excess HCl were then reused 11 times without deteriorating the reaction rate and product purity. Altogether, 22 industrial pigments and 3 azo compounds were synthesized and the reaction can produce up to 22.7 g of product in the laboratory. Some reactions require less than 2 equiv. of HCl and a mechanism explaining this is proposed. In addition, an o-nitro group effect is advanced to explain the differences in coupling reaction rates for o-, m- and p-nitroaniline.


 Please wait while we load your content...
Please wait while we load your content...