Vitamin B12 inhibits α-synuclein fibrillogenesis and protects against amyloid-induced cytotoxicity†
Abstract
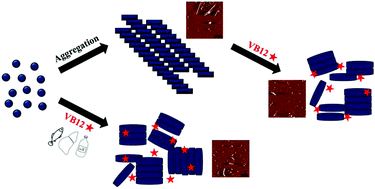
Vitamin B12 (VB12) is a necessary micronutrient for growth and the development of the nervous system. Severe deficiencies of VB12 have been linked to damage of learning and memory ability and cognitive decline, along with several neurological diseases. Misfolding and aggregation of α-synuclein (αSN) into insoluble fibrils is associated with the onset and progression of Parkinson's disease (PD), which affects tens of millions of elderly patients all over the world. Developing novel inhibitors to obstruct the aggregation of αSN has become a topic of intense research. In this study, the inhibitory effect of VB12 against the fibrillogenesis and cytotoxicity of αSN was systematically analyzed using thioflavin T (ThT) fluorescence, atomic force microscopy (AFM), circular dichroism (CD) spectroscopy and cytotoxicity assays. Both ThT and AFM results showed that VB12 could inhibit αSN fibrillogenesis in a dose-dependent manner. CD data suggested that VB12 delays the conformational conversion of αSN to β-sheet rich structures, especially to the parallel β-sheet conformation. As a result, VB12 greatly alleviated the cytotoxicity of αSN aggregates. Moreover, VB12 was also found to disassemble preexisting mature αSN fibrils and attenuate the consequent cytotoxicity. These findings not only provide a comprehensive understanding of the inhibitory effect of VB12 on αSN fibrillogenesis, but also identify a valuable nutrient source that possesses great potential to be developed as a new functional food ingredient to help alleviate PD.


 Please wait while we load your content...
Please wait while we load your content...