Structural transformation of sulfidized zerovalent iron and its impact on long-term reactivity†
Abstract
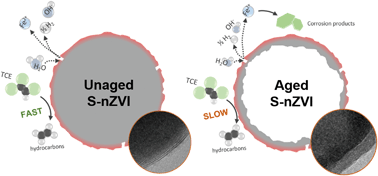
Sulfidized nanoscale zerovalent iron (S-nZVI), synthesized via two-step synthesis using Na2S, is an emerging in situ material for groundwater remediation, composed of a metallic iron core and iron sulfide shell. The shell efficiently transfers electrons from the core to its surface for contaminant reduction, while simultaneously protecting the core from anoxic corrosion. However, what controls the S-nZVI longevity is poorly understood. In this study, we characterized at high resolution the structure of S-nZVI and assessed its reactivity with trichloroethene (TCE) with increasing aging. Our data of freshly synthesized material show that the S-nZVI shell primarily consists of ∼5 nm-thick nanocrystalline mackinawite (FeSm) with structural imperfections and heterogeneous crystal orientations. As S-nZVI was aged in anoxic artificial groundwater for up to 180 days, the shell remained mostly intact, while the iron core significantly corroded, resulting in hollow particle structures. We interpret that FeSm defects caused the deterioration of the core. Between 0 and 120 days of aging, rate constants for TCE reduction decreased by only ∼41%. This shows that FeSm remained accessible for TCE reduction; but as the core became depleted, the reduction rate decreased. Re-spiking experiments with TCE oxidized ∼1/4 of the core while the FeSm structure was unaffected. This indicates that the FeSm does not oxidize during TCE reduction, but merely transfers the electron from the core. Overall, these results demonstrate that S-nZVI is able to sustain its reactivity over extended periods due to the persistence of FeSm against oxidation, while its defects control the extent of core corrosion.


 Please wait while we load your content...
Please wait while we load your content...